Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of isotopes and their significance in understanding the diversity of atoms.
ii. Differentiate between isotopes of the same element based on their neutron numbers and mass numbers.
iii. Understand the concept of stable isotopes and radioactive isotopes, and explain their implications for atomic stability and decay.
iv. Identify and compare the properties of isotopes of hydrogen (H), carbon (C), chlorine (Cl), and uranium (U), highlighting their unique characteristics.
v. Apply the concept of isotopes to real-world applications, such as radioisotope dating and medical imaging.
Introduction
The realm of atoms, the fundamental building blocks of matter, is not as uniform as one might imagine. Within the world of atoms, there exists a fascinating diversity, known as isotopes. Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei.
i. Isotopes: Atoms with a Unique Neutron Signature
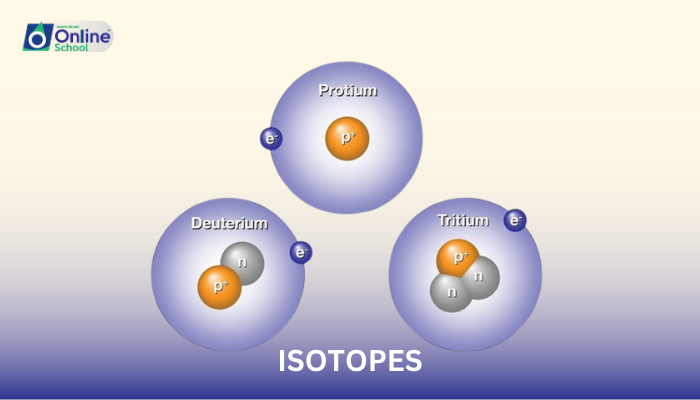
While atoms of the same element share the same number of protons, their neutron numbers can vary, giving rise to isotopes. For instance, hydrogen has three isotopes: protium (1 proton, no neutrons), deuterium (1 proton, 1 neutron), and tritium (1 proton, 2 neutrons).
ii. Comparing Isotopes: A Tale of Mass Numbers
The mass number of an atom is the sum of its protons and neutrons. Since isotopes of the same element have the same number of protons, their mass numbers differ due to variations in neutron numbers. For example, the mass numbers of protium, deuterium, and tritium are 1, 2, and 3, respectively.
iii. Stable Isotopes: Atoms in Harmony:
Most isotopes are stable, meaning their nuclei remain intact and do not decay spontaneously. These stable isotopes form the majority of the atoms we encounter in everyday life.
iv. Radioactive Isotopes: Atoms in a State of Flux
A small fraction of isotopes are radioactive, meaning their nuclei undergo spontaneous decay, emitting radiation in the form of particles or energy. This decay process leads to the transformation of the radioactive isotope into a different atom or isotope.
Isotopes in Action: From Dating to Imaging
Isotopes play crucial roles in various fields:
Radioisotope Dating: The decay rates of radioactive isotopes provide a method for determining the age of ancient artifacts and geological formations.
Medical Imaging: Radioactive isotopes are used in techniques like PET scans and nuclear medicine to visualize internal structures and diagnose medical conditions.
Exploring Isotopes of Familiar Elements
Let's explore the isotopes of some familiar elements:
Hydrogen: Protium, deuterium, and tritium. Deuterium, also known as heavy hydrogen, is used in nuclear reactors. Tritium is used in luminous paints and as a tracer in biological research.
Carbon: Carbon-12, carbon-13, and carbon-14. Carbon-13 is used in NMR spectroscopy to study molecular structures. Carbon-14 is a radioactive isotope used in radioisotope dating.
Chlorine: Chlorine-35 and chlorine-37. Chlorine-35 is the most abundant isotope of chlorine. Chlorine-37 is a radioactive isotope used in groundwater tracing.
Uranium: Uranium-235 and uranium-238. Uranium-235 is the fissile isotope of uranium, used in nuclear reactors and weapons. Uranium-238 is the most abundant isotope of uranium and is not fissile.
Isotopes, with their unique neutron signatures, add a layer of complexity and diversity to the atomic world. They play a vital role in various fields, from dating ancient artifacts to diagnosing medical conditions. Understanding isotopes is essential for comprehending the intricacies of matter and the diverse applications of atoms in our world.